Liquid metals for energy storage
Reliable and economical systems for storing large amounts of energy are needed for industrial nations such as Germany to succeed in making strides toward a regenerative energy supply. An international team led by Tom Weier and Norbert Weber from the Institute of Fluid Dynamics at the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) aims to bring such applications to a level in which they are ready for use. The SOLSTICE project strives to develop energy storage systems based on liquid sodium and zinc from January 2021 onwards. The European Union is funding the project with eight million Euros through the Horizon 2020 program.
Liquid metals and molten salts heated to several hundred degrees Celsius and separated only by a semi-permeable membrane could lead energy intensive industries into the regenerative age. Their purpose is to store energy from the sun and wind on a large scale and provide it at night or in unfavorable weather conditions. Scientists from nine research institutes and three companies have now joined forces to make the required technology ready for use.
“Developing efficient energy storage for industrial applications is a hot topic,” says Dr. Tom Weier from the HZDR’s Magnetohydrodynamics department. “With the phase-out of fossil and nuclear power, storage systems will be required to balance energy supply and demand.” Electricity from renewable sources is not only subject to seasonal fluctuations, but also to variations between daytime and nighttime. Periods of low renewable energy supply are today buffered by conventional power plants. When these plants will be shut down in the coming years, other solutions must be found. Long-term storage based on regeneratively produced hydrogen or methane are suitable for periods of several months. Short-term storage systems based on battery technology, however, are more appropriate for bridging the night.
“Until now, there hasn’t been a very convincing solution,” says Weier. “Systems with lithium-ion batteries work in principle, but would be costly in terms of resources on the industrial scale. “This is because lithium is limited in supply and concentrated deposits are scarce.” Lithium-ion batteries today also consist of many small cells. “The active material that actually stores the energy is packaged in small portions,” explains Weier. “In addition, interconnections and control units are needed to build batteries and battery stacks. Overall, this requires a great deal of construction material.”
Sodium instead of lithium
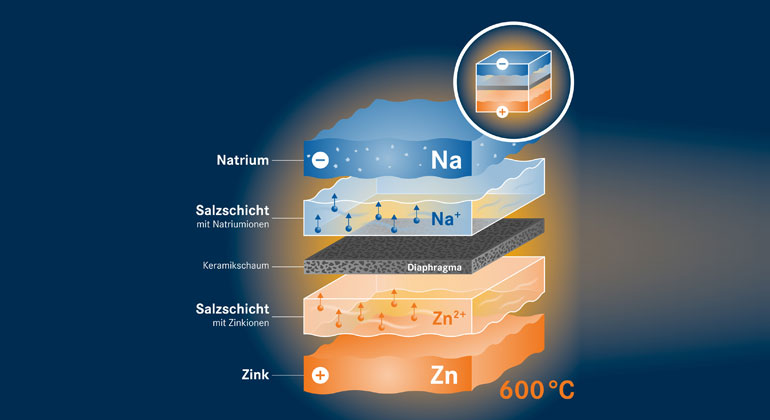
This is why he and his colleagues are forging a different path. “We are using sodium and zinc as the active materials,” explains his colleague Dr. Norbert Weber. There are highly practical reasons for doing so. Sodium is the sixth most common element on the planet and is available in large quantities. As a component of various salts, for example, approximately eleven grams of the alkali metal are dissolved in every liter of seawater. Zinc is actually rarer but zinc resources available worldwide are still immensely abundant. While lithium needs to be imported – today mainly from China, Australia or Chile – Europe possesses its own active zinc mines. That, according to Weber, would greatly reduce the dependence of European energy projects on other countries.
In their research project, the scientists have two different systems in mind. One is to work at 600 degrees Celsius, while the other will operate at 300 degrees Celsius. “In the first system, both the electrodes and the electrolyte are liquid,” says Weber, describing the composition. “Our Norwegian partners have already experimented with this setup and have verified that the principle works. Energy in the megawatt hour range is to be stored here, which predestines such batteries for industrial applications.”
The liquid metals serve as battery electrodes in the second system as well. The electrolyte, on the other hand, is solid. “Our partners in Switzerland already have functional systems that work with nickel chloride. We want to replace the nickel chloride with zinc chloride in the project,” the expert explains. “These batteries can even be envisioned as home storage units in the kilowatt hour range.” Within the coming four years, the scientists plan to develop both systems to the extent that they can demonstrate their functionality in a realistic environment. Given that the second system builds largely on proven technology, there is a good chance to bring it close to the market during the run-time of the project.
Contributing to the energy transition
The HZDR is in charge of not only project coordination, but also construction of the battery cells. “We already have many years of experience in dealing with large amounts of liquid sodium at the Institute of Fluid Dynamics,” says Weier, referring to the DRESDYN infrastructure project in which comprehensive tests with liquid metals are undertaken. “In addition to application, research of course also plays a vital role in the SOLSTICE project,” says Weber. “We want to understand precisely what processes take place in our storage systems.”
The two scientists are certain that their approach can make an important contribution to the energy transition. “Our advantage is the extremely simple design,” says Weier. “This makes the battery cells very scalable. Together with the cheap active materials, we could therefore achieve a significantly lower system price than with other electrical energy storage systems.”