New material could capture toxic gases from atmosphere
An international team of scientists has developed a material that can remove nitrogen dioxide gas and other toxic greenhouse gases from the atmosphere.
This discovery could lead to air filtration technologies that cost-effectively capture and convert large quantities of gases, reducing pollution and global warming.
The research, which was led by The University of Manchester, is being published in Nature Materials. The discovery was confirmed using neutron scattering technique at the United States Department of Energy’s Oak Ridge National Laboratory (ORNL).
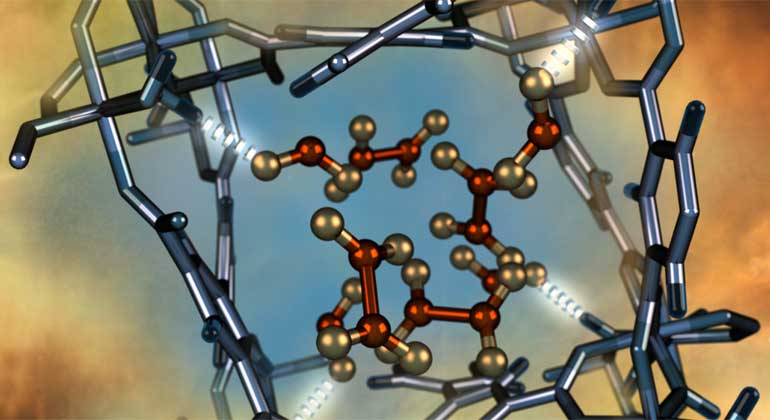
The material, which is called MFM-300(Al), is a metal-organic framework (MOF). MOFs are a class of porous crystalline materials that can act as sponges to trap gases in order to purify and separate them.
The MOF developed by the research team is the first of its kind to exhibit selective, fully reversible and repeatable capability to remove nitrogen dioxide gas from the atmosphere. Simply put, this means that the material can take away and store toxic gas molecules over and over again, which was previously not possible.
Capturing greenhouse and toxic gases from the atmosphere is a challenge due to their relatively low concentrations in the atmosphere and the presence of moisture in the air. This can negatively affect separating targeted gas molecules from other gases. Another challenge is finding a practical way to release a captured gas. MOFs offer solutions to many of these challenges.
Dr Sihai Yang, one of the study’s lead authors and a lecturer in inorganic chemistry at the University’s School of Chemistry, said: “Despite the highly reactive nature of nitrogen dioxide, our material proved extremely robust.
“Other studies of different porous materials found that they were unstable and decomposed with nitrogen dioxide, or that the regeneration process was too difficult and costly.” Professor Martin Schröder, Vice-President and Dean of the Faculty of Engineering and Physical Sciences
“It is the first example of a metal-organic framework that exhibits a highly selective and fully reversible capability for repeated separation of nitrogen dioxide from the air, even in presence of water.”
Professor Martin Schröder, another lead author and Dean of the Faculty of Science and Engineering, added: “Other studies of different porous materials found that they were unstable and decomposed with nitrogen dioxide, or that the regeneration process was too difficult and costly.”
As part of the research, the scientists used neutron scattering techniques to confirm how the material captures nitrogen dioxide molecules, allowing it to remove them from the atmosphere. This was done at United States’ Department of Energy’s ORNL in Tennessee.
Timmy Ramirez-Cuesta, a co-author from ORNL’s Neutron Sciences Directorate added: “Neutrons can easily penetrate dense material and they are sensitive to lighter elements, such as the hydrogen atoms inside the MOF, which enabled us to observe how the nitrogen dioxide molecules bind to the porous sponge which contains nano-sized pores,”
The ability to directly observe how and where MFM-300(Al) traps nitrogen dioxide is helping the researchers validate a computer model of gas separation processes using MOFs. This could help identify how to produce and tailor other materials to capture a variety of different gases.
Yongqiang Cheng, an ORNL neutron scattering scientist and co-author, said “Computer modeling and simulation played critical roles in interpreting the neutron scattering data. Our goal is to integrate the model with experimental techniques to deliver results that are otherwise difficult to achieve.”
- Advanced materials is one of The University of Manchester’s research beacons – examples of pioneering discoveries, interdisciplinary collaboration and cross-sector partnerships that are tackling some of the biggest questions facing the planet. #ResearchBeacons
- The paper ‘Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework’ has been published in Nature Materials.