Producing ‘green’ energy — literally — from living plant ‘bio-solar cells’
Though plants can serve as a source of food, oxygen and décor, they’re not often considered to be a good source of electricity. But by collecting electrons naturally transported within plant cells, scientists can generate electricity as part of a “green,” biological solar cell.
Now, researchers reporting in ACS Applied Materials & Interfaces have, for the first time, used a succulent plant to create a living “bio-solar cell” that runs on photosynthesis.
In all living cells, from bacteria and fungi to plants and animals, electrons are shuttled around as part of natural, biochemical processes. But if electrodes are present, the cells can actually generate electricity that can be used externally. Previous researchers have created fuel cells in this way with bacteria, but the microbes had to be constantly fed. Instead, scientists, including Noam Adir’s team, have turned to photosynthesis to generate current. During this process, light drives a flow of electrons from water that ultimately results in the generation of oxygen and sugar. This means that living photosynthetic cells are constantly producing a flow of electrons that can be pulled away as a “photocurrent” and used to power an external circuit, just like a solar cell.
Certain plants — like the succulents found in arid environments — have thick cuticles to keep water and nutrients within their leaves. Yaniv Shlosberg, Gadi Schuster and Adir wanted to test, for the first time, whether photosynthesis in succulents could create power for living solar cells using their internal water and nutrients as the electrolyte solution of an electrochemical cell.
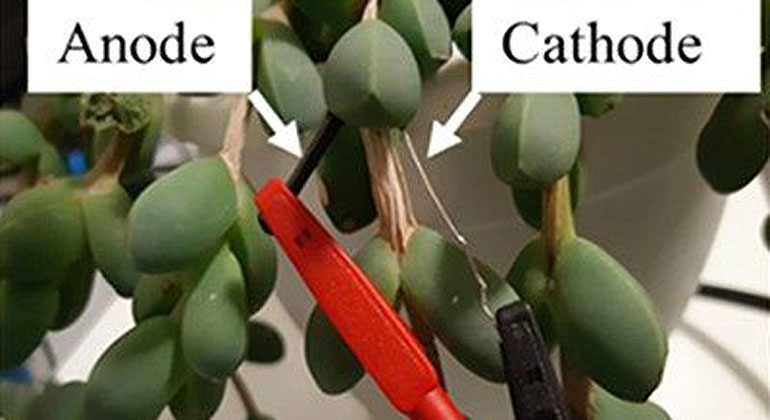
The researchers created a living solar cell using the succulent Corpuscularia lehmannii, also called the “ice plant.” They inserted an iron anode and platinum cathode into one of the plant’s leaves and found that its voltage was 0.28V. When connected into a circuit, it produced up to 20 µA/cm2 of photocurrent density, when exposed to light and could continue producing current for over a day. Though these numbers are less than that of a traditional alkaline battery, they are representative of just a single leaf. Previous studies on similar organic devices suggest that connecting multiple leaves in series could increase the voltage. The team specifically designed the living solar cell so that protons within the internal leaf solution could be combined to form hydrogen gas at the cathode, and this hydrogen could be collected and used in other applications. The researchers say that their method could enable the development of future sustainable, multifunctional green energy technologies.
The authors acknowledge funding from a “Nevet” grant from the Grand Technion Energy Program (GTEP) and a Technion VPR Berman Grant for Energy Research and support from the Technion’s Hydrogen Technologies Research Laboratory (HTRL).
The American Chemical Society (ACS) is a nonprofit organization chartered by the U.S. Congress. ACS’ mission is to advance the broader chemistry enterprise and its practitioners for the benefit of Earth and all its people. The Society is a global leader in promoting excellence in science education and providing access to chemistry-related information and research through its multiple research solutions, peer-reviewed journals, scientific conferences, eBooks and weekly news periodical Chemical & Engineering News. ACS journals are among the most cited, most trusted and most read within the scientific literature; however, ACS itself does not conduct chemical research. As a leader in scientific information solutions, its CAS division partners with global innovators to accelerate breakthroughs by curating, connecting and analyzing the world’s scientific knowledge. ACS’ main offices are in Washington, D.C., and Columbus, Ohio.