What makes lithium batteries explosive
A new model explains dendrite growth in batteries
Lithium-based batteries are extremely powerful, and potentially highly explosive! When such batteries are recharged repeatedly, something called dendrites may form and can trigger a short circuit, causing the battery to burst into flames. Chemists at Ulm University have now developed a model that explains how and why certain metals form dendrites during deposition. This scientific advance, which is significant in battery research, has been published as a hot paper in the respected journal Angewandte Chemie.
The world needs new, high-performance batteries to master the energy transition and drive electromobility forward. To date, lithium-ion batteries in particular have been powering smartphones, laptops and electric cars. But the performance of these batteries is limited, particularly for the demands of electromobility. The problem here is that, to prevent short circuits, lithium ions are embedded in graphite. This increases the volume and weight of the batteries, lowering the range accordingly. Batteries with a pure lithium electrode would have a significantly higher energy density, but they tend to form dendrites. These branch-like protrusions gradually form at the negative electrode when the battery is charged. When they reach the counter-electrode, these dendrites can interact with flammable electrolytes and cause a short circuit, resulting in the battery catching fire. This phenomenon is not only the subject of multiple YouTube videos, it is also being studied by researchers around the world. However, it is not yet understood why metals such as lithium form dendrites, but others like copper and silver do not. Other materials form these dangerous crystal structures only at very high voltages. But Professor Wolfgang Schmickler and Dr Elizabeth Santos from the Institute of Theoretical Chemistry at Ulm University have now developed a model that explains the formation of the branch-like dendrites.
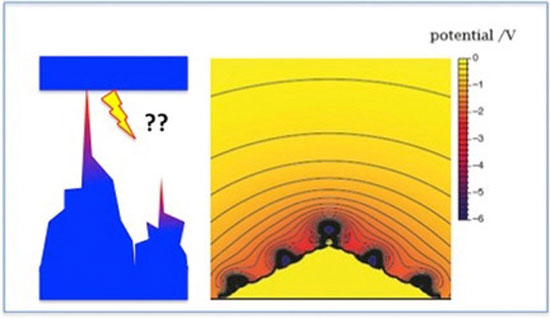
Using the Ulm supercomputer JUSTUS 2, the researchers performed quantum chemical calculations involving a further development of the density functional theory (DFBT+). Their results suggest the following scenario for dendrite formation: Every metal has what is known as a point of zero charge. If the metal is deposited at potentials below this point of zero charge – i.e. at a negatively charged electrode – the crystal-like dendrites are formed. “During deposition, small irregularities like protrusions keep forming on the surface. Following the laws of electrostatics, the negative charge concentrates on the tips of such clusters and attracts the positively charged lithium ions. Thus, these peaks continue to grow and eventually form dendrites,” explains Professor Schmickler. In addition, the researchers were able to demonstrate another phenomenon that contributes to the formation of dendrites: The negative charge reduces the surface tension, promoting the formation of protrusions on the surface. Santos and Schmickler compare this process to dishwashing detergent, which facilitates the forming of bubbles in water.
These findings are compatible with previous research. However, Schmickler and Santos’ calculations are the first to develop a model at the atomic level. This can be applied to other metals, and at the same time it explains why copper, for example, is not at all susceptible to dendrites. “In metals such as copper or silver, the surface is positively charged during deposition. If a small protrusion forms on the surface there, a positive charge accumulates. This repels the positively charged metal ions, and the cluster cannot continue to grow and form dendrites,” explains Dr Elizabeth Santos.
What is the practical relevance of these research findings for the development of high-performance batteries? With their new model, the chemists are able to show why some relevant materials form dendrites and others do not. In addition, they provide an explanation for the formation of crystal structures at the atomic level. “In principle, our model predicts how to avoid the formation of dendrites in rechargeable batteries. However, this would require a solvent that meets conflicting requirements. For the time being, our results are primarily of theoretical relevance,” the authors emphasise. Santos and Schmickler were supported in their research endeavours by the German Research Foundation (DFG) and CONICET, Argentina’s National Scientific and Technical Research Council.
Battery research at Ulm University
Ulm University and the City of Science that surrounds it are considered international leaders in battery research. The breeding ground of this research is the long-standing significance of electrochemistry at Ulm University. Basic and application-oriented research are still intertwined today. In 2011, together with the Karlsruhe Institute of Technology (KIT) and other important partners, Ulm University founded the Helmholtz Institute Ulm (HIU), which specializes in battery research. In addition the KIT, Ulm University and other partners have acquired Germany’s only Cluster of Excellence in the field of battery research, POLiS.
The primary aim is high-performance batteries without the use of lithium and cobalt. The cluster is in turn incorporated into one of the world’s largest platforms for electrochemical energy storage (CELEST).
- Elizabeth Santos, Wolfgang Schmickler: The Crucial Role of Local Excess Charges in Dendrite Growth on Lithium Electrodes | Angewandte Chemie International Edition | Open Access